All API Impurities and Reference Standards
Category starting from letter:
Displaying 1 to 8 ( of 8 products )
Lorazepam EP Impurity A
Lorazepam EP Impurity A
| Catalogue No : | GL-L2902 |
| Common name: | Lorazepam EP Impurity A; Lorazepam USP Related Compound B |
| CAS : | 2958-36-3 |
| Mol. weight | 266.1 |
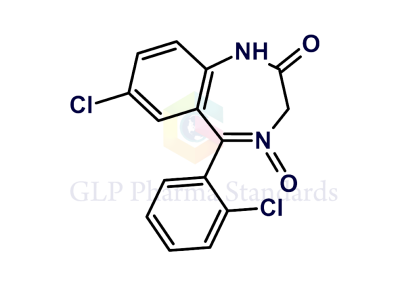
Lorazepam EP Impurity D
Lorazepam EP Impurity D
| Catalogue No : | GL-L2903 |
| Common name: | Lorazepam EP Impurity D |
| CAS : | 54699-91-1 |
| Mol. weight | 321.2 |
Lorazepam EP Impurity C
Lorazepam EP Impurity C
| Catalogue No : | GL-L2904 |
| Common name: | Lorazepam EP Impurity C |
| CAS : | 2955-37-5 |
| Mol. weight | 321.2 |
Lorazepam USP Related Compound D
Lorazepam USP Related Compound D
| Catalogue No : | GL-L2905 |
| Common name: | Lorazepam USP Related Compound D |
| CAS : | 54643-79-7 |
| Mol. weight | 319.1 |
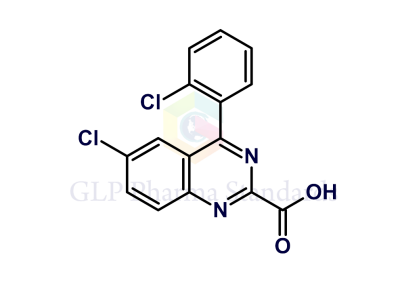
Lorazepam USP Related Compound A
Lorazepam USP Related Compound A
| Catalogue No : | GL-L2907 |
| Common name: | Lorazepam USP Related Compound A; Lorazepam EP Impurity B |
| CAS : | 2848-96-6 |
| Mol. weight | 363.19 |
Lorazepam Related Compound C
Lorazepam Related Compound C
| Catalogue No : | GL-L2908 |
| Common name: | Lorazepam Related Compound C; Lorazepam EP Impurity E |
| CAS : | 93955-15-8 |
| Mol. weight | 303.14 |
Lorazepam Related Compound E
Lorazepam Related Compound E
| Catalogue No : | GL-L2909 |
| Common name: | Lorazepam Related Compound E |
| CAS : | 773871-49-1 |
| Mol. weight | 305.16 |
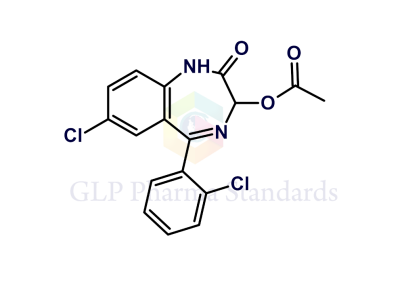
2-Chloro-N-[4-chloro-2-(2-chlorobenzoyl)phenyl]acetamide
2-Chloro-N-[4-chloro-2-(2-chlorobenzoyl)phenyl]acetamide
| Catalogue No : | GL-L2910 |
| Common name: | 2-Chloro-N-[4-chloro-2-(2-chlorobenzoyl)phenyl]acetamide |
| CAS : | 14405-03-9 |
| Mol. weight | 342.6 |




![2-Chloro-N-[4-chloro-2-(2-chlorobenzoyl)phenyl]acetamide 2-Chloro-N-[4-chloro-2-(2-chlorobenzoyl)phenyl]acetamide](https://www.glppharmastandards.com/product_images/optimised/lorazepam_acetanilide_impurity.png)